Even if we see the New Testament as an accurate record of events 2000 years ago, 2000 years ago may feel quite distant to us today. This thinking lead me to the question: is there anything today that allows me to believe in God?
A quick "google" of questions such as "does God exist?" or "proof for God" turns up plenty of results. Some might seem convincing at first, but many of them never really convinced me for long.
The results I found were varied and argued from different angles. Some arguments took a philosophical-physics approach, for example: we now know the universe had a beginning and is bound by natural laws. The laws that govern the universe were required to facilitate its creation but without time, the laws couldn't be pre-existant naturally. In a similar vein, intelligent creation is implied by the fact that the universe always behaves according to the laws of mathematics.
Some purely philosophical arguments I've encountered focus on objective moral standards that are common to civilizations throughout the world and throughout history, despite the fact that the civilizations have existed in complete isolation from each other. Likewise, belief in the supernatural existed in all known civilizations, despite their isolation both geographically and historically, giving rise to the argument that our natural predisposition to belief in a deity comes from the existence of the deity.
Despite the fact I considered the next argument not enough to be a convincing proof on its own, many others do think it's a good argument for the existence of God. It is a common argument which centres on the unlikeliness and complexity of life. Whatever your conclusion, the facts of the argument are fascinating - at least I think so, hence I included it.
Water and Carbon
Water is generally considered a requirement for life, as any life that is based on molecules almost certainly requires some kind of liquid solvent to be able to move those molecules around so it can perform chemical reactions on them in order to release or store energy. Although chemical reactions can take place in gases and solids, reactions in gas require molecules that are volatile enough to be present in large quantities in a gas, and reactions in a solid occur very slowly.
Water has many unique physical and chemical properties that make it well suited to support the complex chemistry required for life. As water has the rare property of expanding when it freezes, it floats to the top of a body of water, and so prevents oceans and lakes on earth from freezing solid by insulating the water below. It can dissolve many substances easily, and so is ideal for facilitating a wide variety of chemical reactions, and it has a high heating capacity, meaning it takes a lot of energy to make water change temperature, which gives Earth its moderate climate.
Water is also the second most common molecule in the universe. Other liquids do exist naturally in the universe, but not in the sort of abundance water does. Most of these liquids don't have many of the other key properties of water that make it so suitable as the basis for life.
Carbon also seems to be a prerequisite for life. It is mid-way between a metal and non-metal, and so can form compounds with most other elements. Most importantly, Carbon can form long stable chains with itself, and as such forms the building blocks of all known life: carbohydrates, fats, proteins and nucleic acids (DNA & RNA).
Fortunately, Carbon is the fourth most abundant element in the universe - after Hydrogen, which fuels our stars, and Helium and Oxygen, in perfect proportions to both form water and have left-over oxygen for respiration. Had the conditions of the early universe not facilitated an abundance of carbon, but skipped to the next most abundant element with metal and non-metal properties, Silicon, the existence of life would be doubtful. Although Silicon, like carbon, can form a variety of compounds, silicon is incapable of forming long chains. Silicon-based molecules of more than two or three silicon atoms are rare, and so the long molecules of life, such as DNA, could not be based on silicon chains. Additionally, silicon compounds are almost all solids in the temperature range that water is liquid, meaning the water-based chemical reactions of life would not be possible in a silicon-based system.
Another key feature of carbon is that its bonds are strong enough to resist environmental stresses, but weak enough to be manipulated by our bodies with enzymes, which is a requirement of any organism's metabolism. The ability of carbon-based molecules to easily store and release energy is a necessary requirement for life, which is simply not found in other elements. Given that silicon is 10% as abundant as carbon, if it were possible for silicon-based life to have evolved, it should have done so alongside carbon-based life.
Even given the rather over-simplified requirements for life we have just discussed, it seems the more we have discovered in the sciences relating to life, the more obvious it becomes that the conditions required of the universe to be able to support life are extremely improbable.
The Sun's Habitable Zone
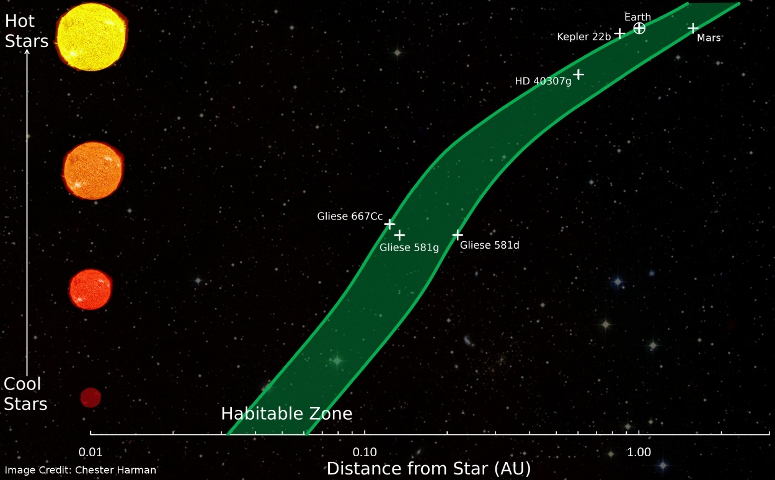
The habitable zone refers to the region around the star in which liquid water can form and remain liquid. The larger and more luminous a star, the further away its planets must orbit to be in the habitable zone.
To make things even more unlikely, the size of the star is important too. Stars that are much larger than the Sun have such short lifetimes that it is unlikely that there would be enough time for any kind of life, particularly complex life, to develop. Having a much smaller star, that would burn for longer, might not help either because planets in the habitable zone of small stars are so close to their star, they are tidally locked: the gravitational attraction that keeps them in orbit around the star causes the planet to always have one face of the planet facing the towards star and the other facing away. This would most likely cause the side facing the star to be too hot for liquid water to exist, and the other side would be too cold.
Our Sun seems to be just the right size to allow life to develop. It is small enough to have a long lifetime, but large enough that a planet can exist in the habitable zone and maintain rapid rotation as it orbits. And talking of orbits, Earth is lucky enough to have an orbit that is very close to circular. If its orbit were slightly more elliptical, the variation of our climate would be too extreme for us to survive.
The Galaxy's Habitable Zone
If it weren't enough for our planet to have to be the perfect distance from a suitably-sized star, with a close to perfectly circular orbit, our solar system lies within the Milky Way's habitable zone.
The centre of the Milky Way is much more dense with stars than the outer regions. Therefore, at the centre, nearby supernova explosions are much more frequent, and the emanated radiation would sterilize any planets in that region of life. Furthermore, the supermassive black hole at the centre of the galaxy emanates intense x-ray radiation, which would also likely destroy any life nearby.
The Milky Way has four major spiral arms. These spiral arms of our galaxy are also densely populated with stars, although not to the same extent as the centre. If our solar system were located in a major spiral arm, in addition to the sterilizing supernovae, it would likely be exposed to stellar interactions that would disrupt the planetary orbits. In other words, life would have very little chance.
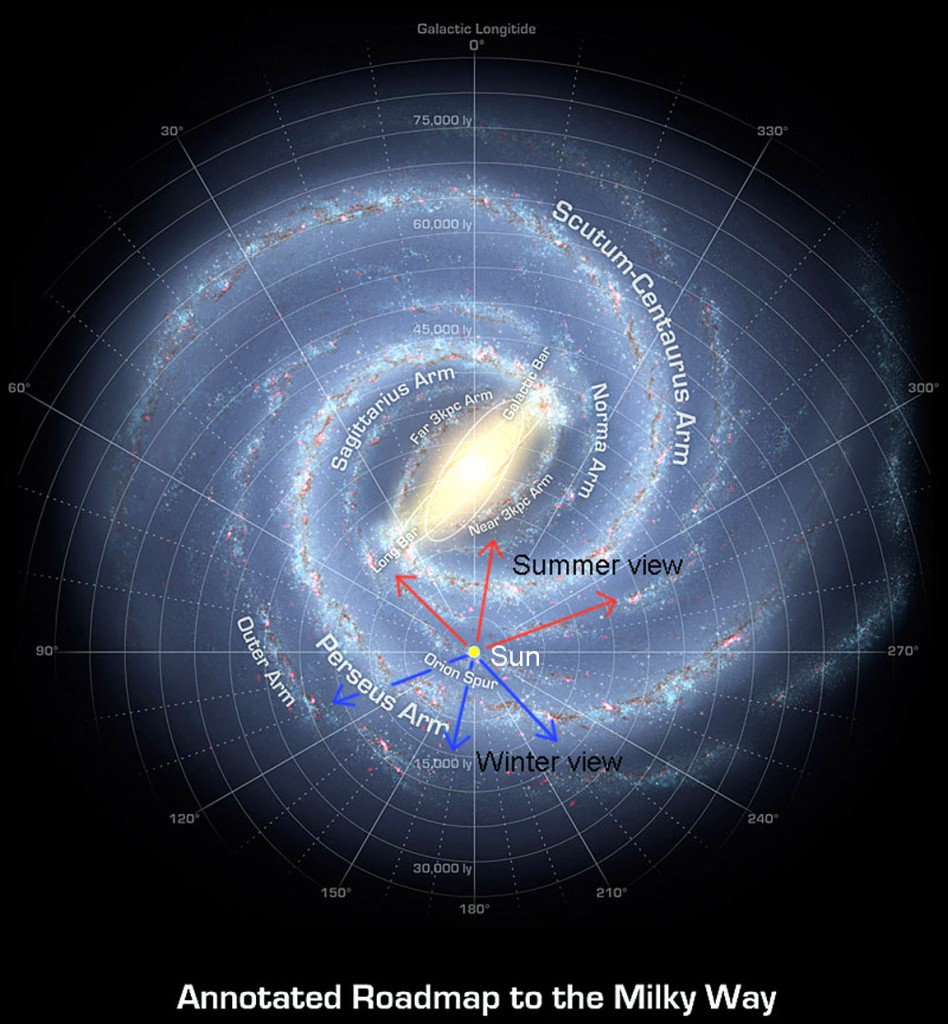
Fortunately for us, our sun, and consequently our solar system, orbits the centre of the Milky Way in the relatively safe region between two spiral arms, at the edge of the Orion Spur. Even more fortunately for us, our sun is unlike most stars in the galaxy, which orbit the centre at a different rate than the spiral arms, and so get swept up by the arms. Instead, our sun lies within a narrow region that means it maintains a stable orbit between two undoubtedly beautiful and awe-inspiring, but hugely destructive spiral arms, in a region known as the galactic co-rotation radius (similar to a geo-stationary satellite that orbits Earth) and so remains equidistant between the arms.
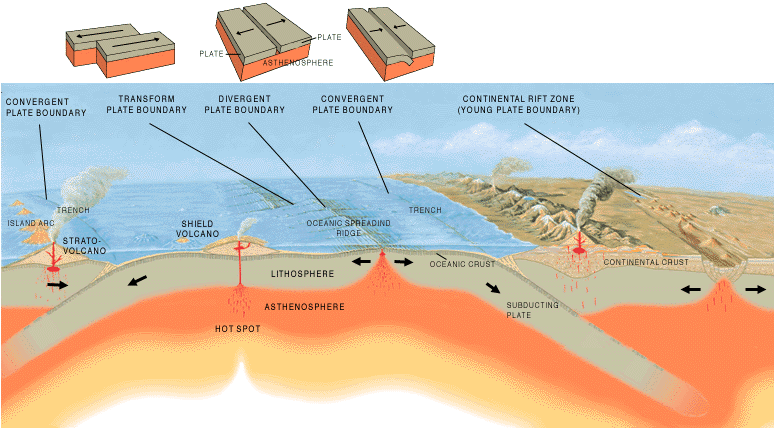
Plate Tectonics
So, our planet lies the perfect distance from a suitably-sized star, with a close to perfectly circular orbit around our sun, which in turn has a stable orbit within the narrow habitable zone of the Milky Way. Even then, we have little guarantee of life.
Normally, we consider earthquakes and volcanic eruptions to be undesirable. However, they have saved the life of every living thing on the planet.
Unlike on Venus and Mars, the crust of the Earth is constantly being recycled, which keeps the carbon dioxide levels in the atmosphere from getting too high or too low. If the levels become too high, as they did on Venus, they act as a greenhouse gas and the planet becomes too hot, liquid water evaporates and the surface of the planet dries up. If the levels become too low, the planet cools and an ice age begins. This has happened several times in Earth's history, but each time, because of the motion of the plates and the continued recycling of the carbon in rocks, carbon was released into the atmosphere, eventually raising the levels of carbon dioxide and allowing the planet to warm again. Without this carbon cycle, planets don't seem to be able to maintain a climate balance appropriate to sustain life.
It is also thanks to volcanic eruptions and their emission of greenhouse gases that we have liquid water. It was previously thought that most planets that contain water, and are in the habitable zone, would go through a phase where the conditions are right to hold liquid water. The phase would occur when a young, dim star starts warming and melts the ice of the planets orbiting it at just the right distance. However, researchers from Peking University in China simulated this transition and found that because of the incredibly high amount of energy required to melt their surface, when the star becomes hot enough to melt the ice, the models quickly transitioned to a greenhouse state: the oceans evaporated and the planets skipped over the habitable phase. Earth was different, because when it thawed, approximately 600 to 800 million years ago, it required less solar heat to melt the ice due to planet-warming atmospheric greenhouse gases emitted by volcanic eruptions.
Luckily for us, we have had just the right amount of volcanic activity: enough to warm the atmosphere to allow liquid water, but not too much that clouds of dust and ash would block out the sun.
Finally, it is also thought that volcanoes are responsible for water being present on Earth in the first place. The evidence suggests water was locked in the minerals within the Earth's mantle, and when the mantle rocks melted, the water dissolved into the magma. As magma rises towards the surface and cools, pressure is reduced, rock crystals form and the water is expelled as steam. Enough steam was produced to fill the oceans and provide an atmosphere. Incidentally, way before we ever thought of or discovered this, Genesis 2:6 said that before it ever rained, there went up a mist from the earth, and watered the whole face of the ground
 .
.
Earth's Magnetic Field Shield
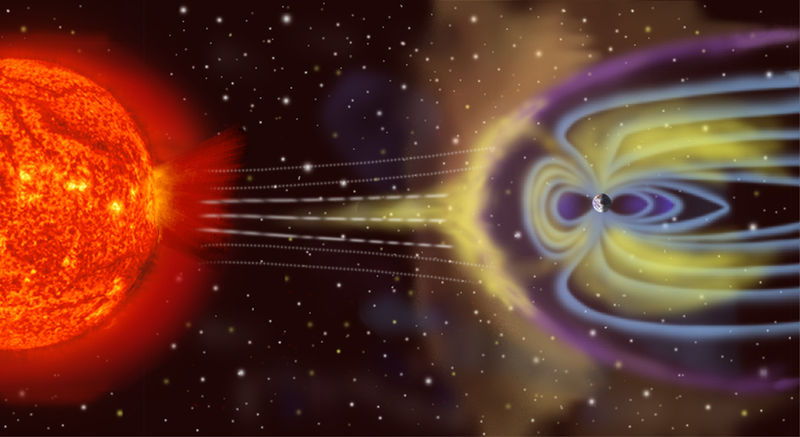
Even given our fortuitous and unlikely position within our solar system and galaxy, and our carbon cycle with just the right amount of volcanic activity, if it weren't for Earth's magnetic field, we certainly would not exist.
Moving electric charges create magnetic fields - this is something we've all seen in school science classes. Scientists think that since the molten material in the outer core of Earth possesses electric charge, its motion is responsible for the production and continuance of our life-protecting magnetic field, which curves outward near the South Pole, turns upward, and re-enters the Earth near the North Pole.
High-energy cosmic rays (principally protons) constantly stream from the sun in all directions at a rate of 400 km/s (or 895,000 mph). Luckily for us, this "solar wind" is deflected by the Earth's magnetic field - although a few collect at our poles, where they impact the upper atmosphere, dissipating their energy and so producing the Northern Lights and Southern Lights. If the magnetic field did not exist, or it was not strong enough, the stream of protons would sweep away our atmosphere, and (not that it would matter to us now, because we have no atmosphere) due to the low pressure, water would evaporate and be swept away into space, too - Earth would look a lot more like Mars.
The Sun's T-Tauri Phase
Today, we are protected from the Sun's solar winds, but at one point in the Sun's history there was a much more violent wind, from which our magnetic field would have been about as protective as a cheap umbrella in a hurricane.
Stars in their early stages are observed to lose a vast amount of matter in one explosive burst. Our sun was likely no exception and blew out a mass equal to two or three present-day Suns. This wind was so fierce that it literally blew away all residual interplanetary gases into outer space. Interplanetary matter was not the only casualty: atmospheres of the planets were also whisked away. However, we still seem to be surrounded by a life-supporting atmosphere of about 20% oxygen and 80% nitrogen, and that's all down to good timing.
Had the explosion happened too early, before the aggregation of matter into planets, the frozen gases held as frost on the surfaces of the dust and rock particles - which were to later cluster and form the planets - would have evaporated and been lost to space, resulting in an Earth without water.
Had the explosion happened too late, after the major volcanic outgassing (where the water locked in minerals within the Earth's mantle was dissolved into magma as the Earth heated and the mantle rocks melted, and was then released as steam as the molten rocks rose to the surface and cooled) the now unlocked water would have been swept away into space by the devastating solar wind, along with the rest of the atmosphere.
Luckily for us, the Sun's T-Tauri phase happened at just the right time: after the planets had formed and Earth had locked its water into its rocks, and before the water was released by volcanic activity.
Jupiter
Even considering the remote likelihood of getting this far, if it weren't for our neighbour, Jupiter, we wouldn't be here now. Because Jupiter is so much more massive than all the other planets, it attracts many asteroids, comets and other objects that travel within the Solar System. This is life-saving because otherwise some of these objects would end up crashing into Earth, and many did in the very early formation of the Solar system. Jupiter's gravity, along with Earth's atmosphere, combine to protect the Earth from many impacts that would certainly have sterilized Earth many times over.
Was Life Inevitable in our Universe?
The chance of life being able to survive on a given planet appears to be remote at best. However, there are billions and billions of planets in the universe.
If I pick 6 numbers from 49 in a lottery, the chance of those numbers being drawn is 1 in 13,983,816 - not good. However, if seven million people played (and each picked a different set of 6 numbers) the chance of someone winning is about 1 in 2 (50%). Similarly, if only one planet was born, the chance of the conditions being right to create life would be minimal, but as the number of planets increases, so does the chance of one of them having the winning conditions.
Writing all this down lead me to a nice realisation for the age-old question: Why is our universe so big? The answer: "because it needed to be". Given the laws that govern the universe, the chance of complex life occurring is so remote that it seems to be essential for the universe to be so large, in order to increase the probability of us occurring somewhere and give us a chance of happening. Obviously, our necessity didn't precede the requirement, but it is a nice realisation.
Was our Universe Inevitable?
Even though the size of the universe may have given us - or any life - a better chance of happening, our universe is a one-shot process.
Unlike it was previously thought, the universe is not infinite, nor does it expand and contract before expanding again, ad infinitum. The universe came from nothing and is expanding at an ever increasing rate, but one day, when the temperature drops to 0 °K, all matter will have stopped interacting and the universe will be dead. So we are very lucky we happened on the first (and only) go!
Does this Mean there is a God?
The unlikeliness of life is often used as an argument for God. But, can it ever be a proof of God's existence? Although it just so happens that we are here and have developed the reasoning to allow us to ask questions such as why are we here?
, if the universe were a slightly different place, we simply would not be here to ask those questions. So, although I like this argument (mostly because I find it fascinating), we must look elsewhere for evidence for the existence of God.